Determination of Hexanal in Foods Utilizing Dynamic Headspace GC/MS
- Home
- Determination of Hexanal in Foods Utilizing Dynamic Headspace GC/MS

Determination of Hexanal in Foods Utilizing Dynamic Headspace GC/MS
INTRODUCTION
Hexanal is one of many well-documented aromatic components that contribute to flavour and aroma in common consumer food products containing omega-6 fatty acids. Hexanal content is also used to measure the oxidative status of foods rich in omega-6 fatty acids.
Dynamic headspace sampling differs from static headspace sampling in that the headspace above the sample is swept with a gas onto an adsorbent trap, rather than allowing a sample and its headspace to reach a static equilibrium before transferring the volatile compounds to a gas chromatograph/mass spectrometer (GC/MS). Employing dynamic headspace sampling provides all the benefits of static headspace with the low-level detection capabilities similar to analysis by purge and trap.
The hexanal content of two common consumer food products, rice and infant formula, was evaluated for hexanal content. Dynamic headspace was used to concentrate hexanal from food products, minimizing labour intensive solvent extraction and clean up. The hexanal was then separated from other volatile compounds and quantitated with a GC/MS.



Omega-6 fatty acids are the most commonly found fatty acid present in the foods that we eat. Hexanal is formed when these omega-6 fatty acids are oxidized. Hexanal is an aromatic component that contributes to flavour and aroma in common consumer food products containing fatty acids. Hexanal content can be used to measure the quality of flavour and aroma of foods rich in fatty acids, as well as their oxidative status.
Rice and infant formula, fatty acid containing consumer food products, were evaluated for hexanal using the dynamic option of the SCION Instruments HT3 Static and Dynamic Headspace Analyzer. The dynamic option improves sensitivity over the static method by continually sweeping and concentrating analytes onto an adsorbent trap resulting in a 10 to 100 fold increase in sensitivity, depending on the compound.
When measuring hexanal content as a lipid oxidative marker, matrix matching is critical because of the effects of different lipid concentrations on hexanal recovery. Elisia1 and García-Llatas2 used matrix matching to calculate the accuracy of their method to monitor lipid oxidation during human milk and infant formula storage. Wang3 used hexanal-free rice for their calibration curve to perform a similar matrix match.
STANDARD AND SAMPLE PREPARATION
Hexanal standards were prepared at 2, 10, 20, 100 and 200 ng/µL in methanol. Calibration curves were created from 10 ng to 1000 ng in 22 mL headspace vials by adding 5 µL of each stock standard to either standard vials or sample vials.
White rice was obtained locally. The rice was lightly ground in a laboratory mill. The rice sample consists of 1 g ground rice for both the calibration curve and samples. The rice standards and samples were evaluated with dynamic headspace at 30 °C. Seven samples of rice were prepared to determine reproducibility and accuracy.
Three sets of rice samples were tested to evaluate volatile released during cooking by adding water. The first set was the dry rice. The second set contained 100 µL of additional water or 10%(v/w) water. The final set contained 500 µL of additional water or 50%(v/w) water.
Infant formula was obtained locally. The infant formula consists of 1 mL for both the calibration curve and samples. The infant formula standards and samples were evaluated with dynamic headspace at 37 °C. Seven samples of infant formula were prepared to determine reproducibility and accuracy.
Table 1. Instrumentation operating conditions
| Parameter | Specification |
|---|---|
| Injector | Split 30:1, 200 °C |
| Column | Rtx – 502.2 |
| Over program | 40°C (2 min), 7°C/min to 120°C, 15°C/min to 240°C (7 min) |
| Carrier | Helium |
| Flow | 1.0 ml/min |
| Software | MSWS/ HT3 Teklink |
| MS Transfer line temperature | 240°C |
| Ion Source | 230°C |
| Ionization Mode | EI |
| Scan start | 1.0 |
| Scan mode | Full scan |
| HT3 | |
| Oven temperature | 150°C |
| Transfer line temperature | 160°C |
| Sample temperature | 37°C |
| Sample equilibrium | 10 min |
CALIBRATION RESULTS
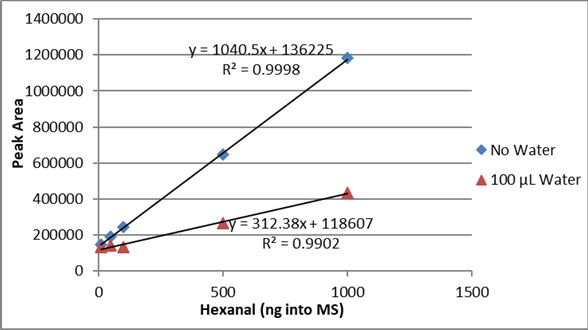
The hexanal curves from 10 ng to 1000 ng of standard and spiked rice including the correlation coefficient (r2) are shown in Figure 2. The hexanal standard curve from 10 ng to 1000 ng in water and infant formula including the correlation coefficient (r2) are shown in Figure 3. The difference between the slopes of the lines confirms that matrix matching must be performed when testing hexanal in rice or infant formula samples.
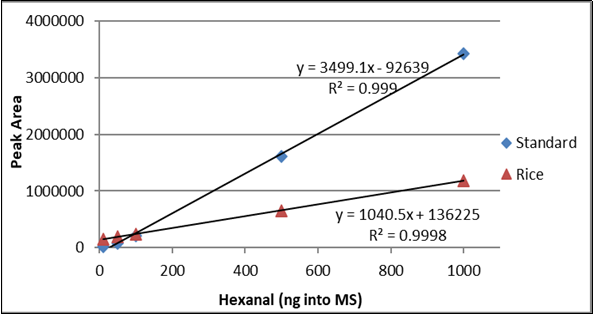
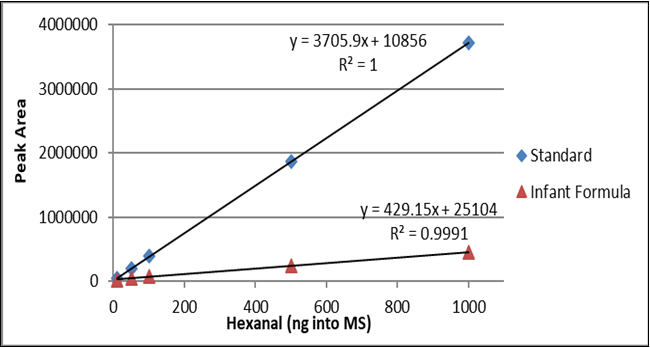
Table 2. Hexanal Concentration for seven samples
| Hexanal Concentration | ||
|---|---|---|
| Sample | Rice (ng/g) | (Formula ng/ml) |
| 1 | 89.3 | 41.8 |
| 2 | 82.8 | 48.6 |
| 3 | 88.4 | 42.8 |
| 4 | 88.1 | 39.3 |
| 5 | 107.4 | 44.5 |
| 6 | 83.5 | 47.1 |
| 7 | 94.3 | 43.8 |
| %RSD | 9.2 | 7.2 |
| Average | 90.5 | 44.0 |
REPRODUCIBILITY AND ACCURACY RESULTS
The concentration of hexanal was calculated as ng/mL for the 7 infant formula samples using the response factor from the calibration data. The concentration of hexanal was calculated as ng/g for the 7 unspiked rice samples using the response factor from the calibration data using rice as a spiking matrix. The fragment ion 56 m/z was used for the calculation. Table 3 shows the data for the seven samples along with the %RSD and the average.
Figure 4 is the comparison of the 56 m/z mass ion chromatogram of a 10 ng hexanal standard in water to an unspiked infant formula sample.
Figure 5 is the comparison of the 56 m/z mass ion chromatogram of a 50 ng hexanal standard to an unspiked rice sample.
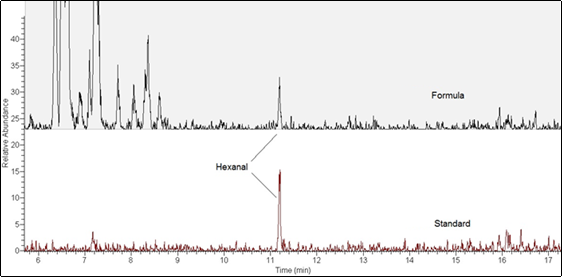
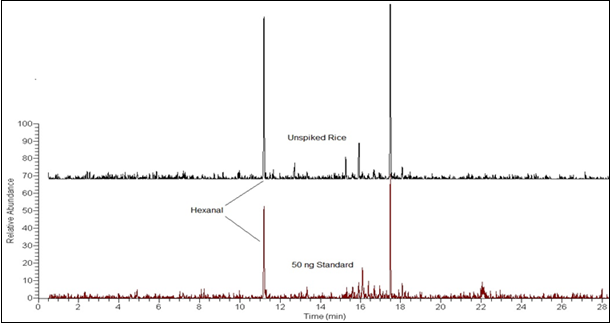
RICE WATER ADDITION RESULTS
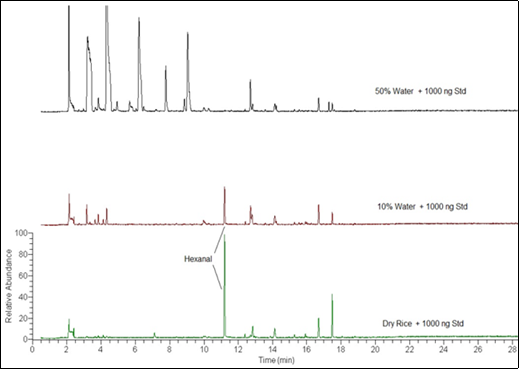
The addition of water to rice samples to evaluate other volatiles released during cooking produced unexpected results with the specified dynamic headspace parameters. The first was the appearance of other volatile compounds that were not detected in the dry rice.
The second was the reduction of hexanal with increasing water content, including the added hexanal standard. Figure 6 compares the total ion chromatogram (TIC) of the dry rice, and rice with 10% (100 µL) and 50% (500 µL) of added water, all with 1000 ng hexanal added.
This reduction of the hexanal peak is also evident in the calibration curves. Figure 7 compares the calibration curve for the dry rice and the 10% added water. The 50% water/rice samples had no detectable hexanal peak and no calibration curve was calculated.
CONCLUSION
Hexanal was readily determined with dynamic headspace analysis in both dry rice and infant formula using the parameters in this evaluation. The calibration curves must use matrix matching to provide accurate data.
The addition of water to the dry rice did increase the release of other volatile compounds. But it also results in the reduction of hexanal, depending on the amount of water added. The mechanism for this loss was not evaluated for this poster.
REFERENCES
1 –Elisia I, Kitts DD, Quantification of hexanal as an index of lipid oxidation in human milk and association with antioxidant components, Journal of Clinical Biochemistry and Nutrition, Volume 49, Issue 3, 2011, pg 147-152
2 – García_Llatas G, Lagarda MJ, Romero F, Abellán P, Farré R, A headspace solid-phase micro extraction method of use in monitoring hexanal and pentane during storage: Application to liquid infant foods and powdered infant formulas, Food Chemistry, Volume 101, Issue 3, 2007, pg 1078-1086, From the Food and Agriculture Organization of the United Nations web address:
http://agris.fao.org/agris-search/search.do?recordID=US201301124132
3 – Yiru Wang, Jaeho Ha, Determination of Hexanal in Rice Using an Automated Dynamic Headspace Sampler Coupled to a Gas Chromatograph-Mass Spectrometer, Journal of Chromatographic Science, 2012: 00:1-7
- Compartilhar
